About ParagonCare
ParagonCare is comprised of 4 strategic pillars: Specialty Diagnostics, Specialty Devices, Capital and Consumables and Service and Technology
Discover how the MacuMira device is elevating patient care with innovation in Dry AMD
Partnership includes launch in Australia and New Zealand, distributed exclusively by ParagonCare, with additional markets to follow.
Windsor, UK and Calgary, Canada - Keeler Ltd, a global leader in ophthalmic innovation with more than a century of heritage, and MacuMira Medical Devices Inc, a pioneering Canadian company, today announced a global strategic partnership. Under the agreement, Keeler will manufacture the MacuMira device and lead its exclusive distribution in Australia and New Zealand through leading diversified healthcare distributor, ParagonCare, together we are officially launching at the annual RANZCO meeting (Royal Australian and New Zealand College of Ophthalmology). MacuMira will continue to offer the therapy directly in Canada, where it has been available since early 2024. The companies also plan to support distribution in additional markets as MacuMira expands internationally.
Dry age-related macular degeneration (Dry AMD) is the leading cause of vision loss among adults worldwide, with few effective treatment options. MacuMira is a unique medical device that uses precisely delivered microcurrent to stimulate retinal pigment epithelium (RPE) cells, enhancing mitochondrial activity to support cellular energy production and preserve or improve visual function in people living with Dry AMD. MacuMira’s non-invasive retinal microcurrent stimulation device represents a breakthrough approach, designed to restore visual function and independence for patients through a 32-minute, in-clinic therapy.
“Keeler has a proud tradition of advancing eye care with uncompromising quality, craftsmanship and innovation in eyecare,” said Andy Harbidge, Keeler Managing Director. “This partnership marks a transformative moment as we align Keeler’s heritage and reach with MacuMira’s groundbreaking therapy. Together, we will bring new hope to patients and clinicians worldwide.”
MacuMira’s therapy is already established in Canada, where thousands of treatments have been delivered safely since early 2024. Clinical evidence, including a randomized controlled trial published in the International Journal of Retina and Vitreous, has demonstrated meaningful improvements in visual acuity and contrast sensitivity.
“This collaboration with Keeler accelerates our mission to change the trajectory of Dry AMD,” said Justin Sather, CEO MacuMira. “By combining Keeler’s global presence with our novel science, we are redefining how this disease is managed, restoring not only vision but also dignity and independence to patients.”
“We are delighted ParagonCare can support this partnership by exclusively distributing the innovative MacuMira device across Australia and New Zealand” said Carmen Riley, CEO, ParagonCare. “Our aim is to enable eye care professionals with innovative solutions to achieve optimal patient care against progressive vision loss.”
Images and further information are available at www.macumira.com.
Media Contacts
Marketing Team
Keeler Ltd
Email: info@keeler.co.uk
Matt Van De Ven
COO
MacuMira Medical Devices Inc
Email: info@macumira.com
ParagonCare
Kylie Inserra - Head of Marketing, Communications and Branding
kylie.inserra@paragoncare.com.au
Anissa O'Connor – Vision Business Unit Manager - anissa.oconnor@paragoncare.com.au
Interviews with executives from all companies are available upon request.
About Keeler Ltd
Founded in 1917, Keeler has built a legacy of innovation and quality in ophthalmic instruments. With a global presence and a commitment to transforming eye care, Keeler sets the standard for precision and reliability in clinical practice.
About MacuMira Medical Devices Inc
MacuMira is a Canadian medical device company dedicated to restoring vision and improving lives through retinal neurostimulation. With a strong clinical foundation and growing international presence, MacuMira is redefining how Dry AMD is managed worldwide.
About ParagonCare
ParagonCare is one of Asia Pacific’s leading diversified healthcare distributors and manufacturers. Delivering pharmaceuticals, capital equipment, diagnostics, medical consumables, devices and complementary medicines for over 100 years. Today, we are an ASX-listed diversified healthcare leader, making healthcare simpler, smarter, and more accessible.

There has been a transformative merger between Paragon Care Group and Clifford Hallam Healthcare (CH2) to create ParagonCare - a leading healthcare wholesaler, distributor, and manufacturer operating across growing healthcare markets in the Asia Pacific region.
The combined entity has an expanded reach including Hospital Pharmacy, Community Pharmacy, Primary Care, Aged Care and Community as well as a Contract Logistics servicing the healthcare industry.
Our extended customer base has access to a broader range of products and services with the most cost-effective solutions.
Explore more:
Learn more about ParagonCare | CH2 here: www.ch2.net.au
Place orders directly online via ParagonCare Direct: direct.paragoncare.com.au

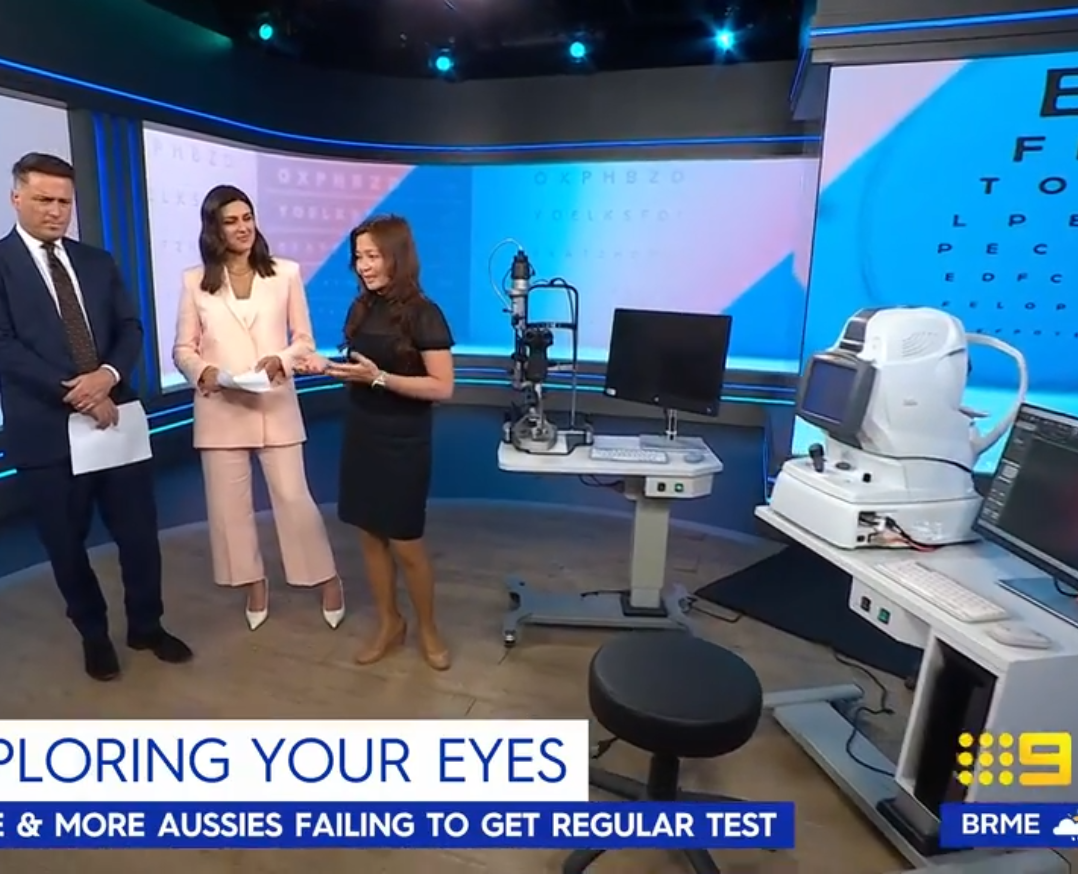
On World Optometry Day 2025, Channel 9’s Today Show featured a special segment with 1001 Optical, showcasing the groundbreaking NIDEK DUO2 OCT in collaboration with ParagonCare | Designs For Vision. This advanced imaging system is revolutionising optometry by providing precise, high-quality images essential for diagnosing and monitoring eye health.
The NIDEK DUO2 OCT, a state-of-the-art imaging system, is designed to provide optometrists with rapid, high-quality retinal and optic nerve imaging, helping to detect and monitor eye conditions with greater confidence. With deep-learning-powered B-scan denoising and RetinaMap technology, it delivers enhanced clarity and diagnostic efficiency while minimising patient movement artifacts. The system also features a built-in 12-megapixel fundus camera, enabling detailed imaging for improved clinical decision-making.
“Early and accurate detection of retinal and optic nerve conditions is essential in modern optometry,” said Marnie Bateman, General Manager of Non-Acute Sales at ParagonCare | DFV. “The NIDEK DUO2 OCT is an important tool that helps optometrists provide high-quality patient care through advanced imaging capabilities. We are thrilled to see this technology showcased on a national platform.”
As a trusted distributor of NIDEK technology in Australia, ParagonCare | DFV is committed to equipping optometry and ophthalmology professionals with state-of-the-art solutions that streamline clinical workflows and improve patient care outcomes. Don’t miss this exciting segment on Channel 9’s Today Show as we explore how cutting-edge technology is shaping the future of optometry.
For more information on the NIDEK DUO2 OCT, reach out to the team.
MT WAVERLEY, VIC – March 12, 2025
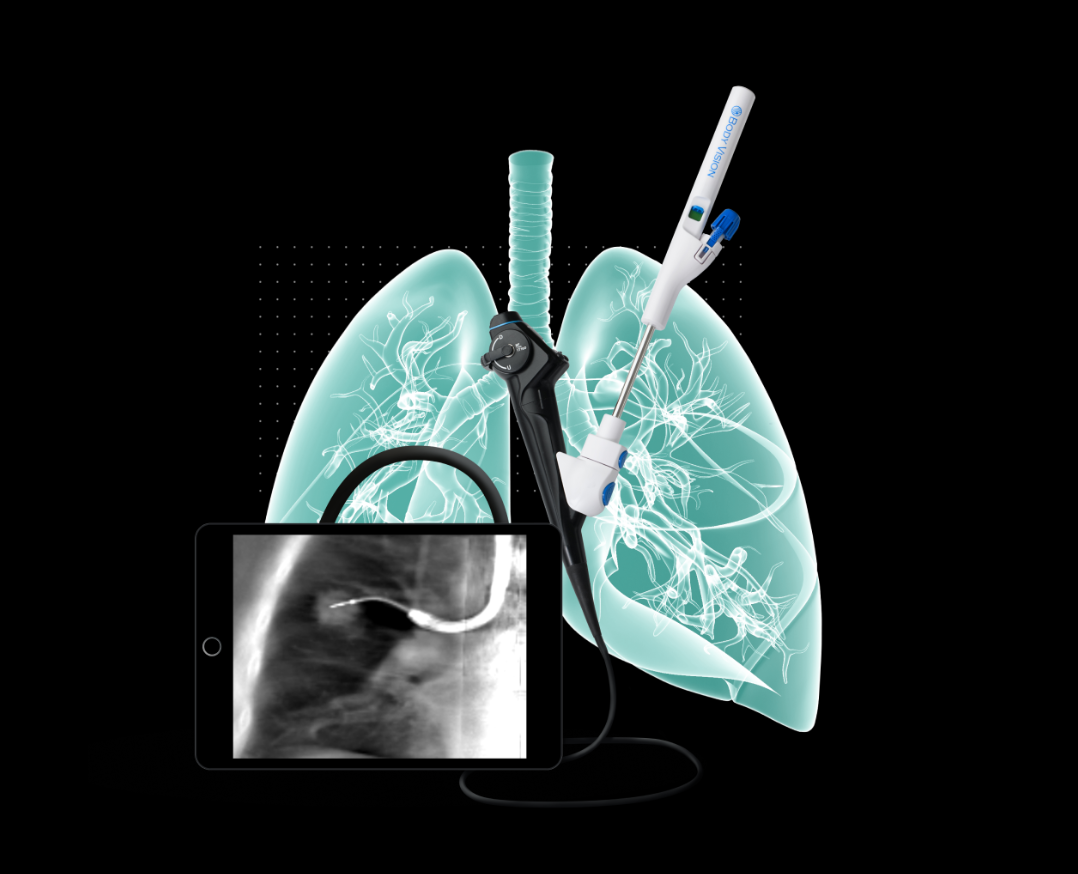
ParagonCare the exclusive distribution partner of BV, is excited to announce that Body Vision Medical’s LungVision® AI-driven advanced imaging system has received regulatory approval from the Therapeutic Goods Administration (TGA) in Australia.
Body Vision Medical’s innovative technology ensures that hospitals and clinics throughout Australia will now have access to state-of-the-art AI-driven imaging solutions, reinforcing the shared commitment to advancing lung cancer diagnosis and treatment.
"We are excited to welcome Body Vision Medical’s technology to our portfolio, as it strengthens our commitment to advancing augmented lung and respiratory medicine through cutting-edge AI. Body Vision's real-time imaging technology offers remarkable opportunities, significantly enhancing the chances for earlier cancer detection and ultimately improves lung cancer survival rates." – Tiffany Chiew, General Manager, Capital and Service.
Lung cancer remains the leading cause of cancer-related deaths in Australia, with an estimated 14,500 new cases diagnosed annually. With the Australian Government Department of Health and Aged Care launching the National Lung Cancer Screening Program (NLCSP) to detect lung cancer at earlier stages, there is an increasing need for a definitive diagnostic pathway for patients with suspicious pulmonary nodules (SPNs).
LungVision®, an AI-driven imaging system that transforms any C-arm into a 3D imaging system, empowers physicians with real-time navigation and enhanced visualisation to achieve more precise and accurate bronchoscopic biopsies. By enabling more confident diagnoses, LungVision® supports the clinical goals of the NLCSP and can enhance patient outcomes. ParagonCare is proud to partner with Body Vision Medical to support this national initiative by providing solutions that align with evolving screening guidelines and clinical practices.
“This approval represents a major step forward in our global mission to transform lung cancer care,” said Matt Baker, CEO at Body Vision Medical. “We are thrilled to partner with ParagonCare to make LungVision® available in Australia and New Zealand, where the need for accurate, early-stage lung cancer diagnosis has never been more urgent.”
ParagonCare will be launching LungVision® to the Australian market at the TSANZSRS the combined meeting for the Thoracic Society of Australia and New Zealand and the Australian and New Zealand Society of Respiratory Science, held from 21st -25th March in Adelaide, South Australia.
We invite you to visit the ParagonCare booth (#54) to meet our team, experience a live demo, and discuss how LungVision® can contribute to the success of the NLCSP and your lung cancer screening efforts.
For more information about LungVision® and its availability in Australia and New Zealand, please contact the ParagonCare team via the ParagonCare website or below contacts.
Visit bodyvisionmedical.com to learn more and connect with us on LinkedIn.
ParagonCare Media Contact:
Kylie Inserra
Group Communications & Branding Lead
T 1300 726 226
E kylie.inserra@paragoncare.com.au
W paragoncare.com.au
ParagonCare Customer Contact:
Tiffany Chiew
General Manager Capital & Service
T 1800 228 118
E tiffany.chiew@paragoncare.com.au
W paragoncare.com.au
MOUNT WAVERLEY VIC - 19 February 2025
In a groundbreaking development for Australian healthcare, ParagonCare has unveiled its strategic partnership with Coreline Soft, an AI-driven, innovative lung cancer screening
software designed to enhance early detection and diagnosis.
Coreline Soft’s comprehensive imaging analysis solutions integrate seamlessly into clinical practice and are transforming diagnosis workflows. Their AI-powered medical imaging solutions are revolutionising how hospitals detect and manage multiple conditions from a single CT scan. This creates opportunities for earlier intervention and potentially better
patient outcomes.
Coreline Soft’s AVIEW product line offers 3D CT image analysis solutions. AVIEW LCS Plus, which can simultaneously screen for the "BIG 3" in lung cancer screening (lung cancer, emphysema, and coronary artery calcification), supports large-scale data analysis and precise nodule detection. It reduces diagnostic variability among medical professionals and is currently supplied to lung cancer screening programs in South Korea, Europe, Germany, Italy, and more.
“By integrating AI-powered healthcare solutions into our portfolio, ParagonCare is reinforcing its commitment to advancing patient care and streamlining radiology workflows. Our partnership with Coreline Soft is a significant step forward in harnessing innovative technology to support healthcare professionals and improve patient outcomes.” – Tiffany Chiew, General Manager, Capital and Service.
"Partnering with ParagonCare is a significant step in expanding access to our AI lung cancer screening solutions across Australia," said Jason Suh, Director of Overseas Business Department at Coreline Soft. "With their deep expertise in medical imaging and strong local presence, ParagonCare is an ideal partner to bring advanced screening technology to more healthcare providers. Together, we aim to enhance early detection, improve diagnostic accuracy, and support better patient outcomes—starting with the NLCSP in July."
ParagonCare is excited to launch the Coreline Soft to the Australian market at the Australian Lung Cancer Conference (ALCC) 2025, held in Adelaide from 19th to 21st February, South Australia.
This year, with the commencement of the National Lung Cancer Screening Program (NLCSP) in Australia, the conference emphasizes the transformative potential of early detection in reducing lung cancer mortality. ParagonCare is proud to partner with Coreline Soft to support this national initiative by providing solutions that align with evolving screening
guidelines and clinical practices.
We invite you to visit the Coreline booth (#3) to meet our team, experience a live demo, and discuss how Coreline Soft can contribute to the success of the NLCSP and your lung cancer screening efforts.
Please contact the ParagonCare team for more information about the innovative Coreline Soft via the website or below contacts.
About ParagonCare
A leader in healthcare technology, devices, software, and consumables, ParagonCare Limited (ASX: PGC) is dedicated to meeting the dynamic needs of the healthcare industry. By harnessing expertise, agility, and strong partnerships, ParagonCare is at the forefront of delivering innovative solutions that improve patient experiences and optimise healthcare workflows.
About Coreline Soft
Coreline Soft is a global leader in AI-powered medical imaging solutions, specialising in advanced CT image analysis for lung cancer screening and comprehensive chest CT interpretation. Driven by innovation and precision, Coreline’s AI technology enhances early disease detection, ensures diagnostic consistency, and optimises radiology workflows. Trusted by national screening programs and leading medical institutions worldwide, Coreline Soft is committed to advancing patient care through cutting-edge imaging technology.
ParagonCare Media Contact:
Kylie Inserra
Group Communications & Branding Lead
T 1300 726 226
E kylie.inserra@paragoncare.com.au
W paragoncare.com.au
ParagonCare Customer Contact:
Tiffany Chiew
General Manager Capital & Service
T 1800 228 118
E tiffany.chiew@paragoncare.com.au
W paragoncare.com.au

Clifford Hallam Healthcare Pty Ltd (ACN 001 655 554) Terms & Conditions of Sale

In accordance with the requirements of the Workplace Gender Equality Act 2012 (Act), please be advised that on 24 May 2024, Paragon Care lodged its annual public report with the Workplace Gender Equality Agency (Agency).
This data is reported in the following documents:
Please refer any questions regarding the content or the finding of this report to hr@paragoncare.com.au

Key Highlights
22/12/2020
Key Highlights
15/04/2021